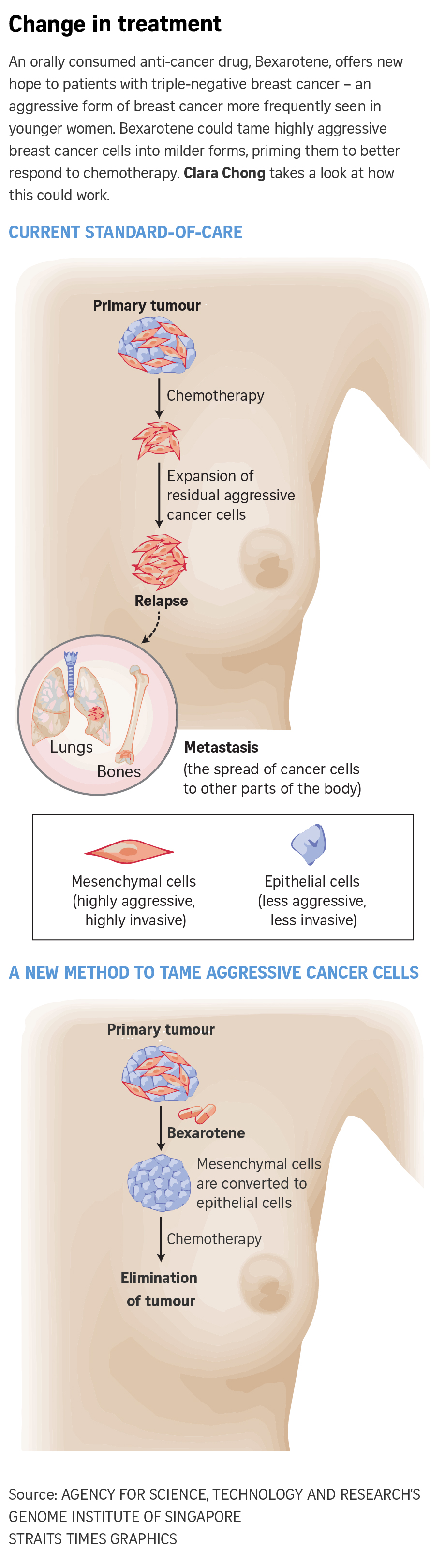
SINGAPORE - An orally consumed anti-cancer drug known as Bexarotene offers new hope to patients with triple-negative breast cancer (TNBC) - an aggressive form of breast cancer that appears more frequently among women below 40.
This drug has been found to be able to tame highly aggressive and invasive breast cancer cells by converting them from a more aggressive cell state (mesenchymal) to a milder one (epithelial). Doing so primes the cancer cells to better respond to chemotherapy and allows for more effective elimination of the tumour and a lower chance of relapse.
A three-year-long clinical trial to test this new method of treatment started last October. So far, four patients have been recruited for the trial. They intend to recruit 12 patients, but this can be increased to 20.
Patients enrolled in the trial, named Bexmet, are given Bexarotene during a lead-in period of two weeks prior to the start of the treatment cycle. Each cycle comprises two weeks of taking the oral chemotherapy drug Capecitabine, followed by one week of Bexarotene.
This cycle is repeated so long as the patient is responsive to treatment.
Patients are expected to undergo a total of four biopsies of the tumour tissue while on-trial: pre-treatment, after the two-week Bexarotene lead-in period, after two weeks of Capecitabine in the first cycle, and upon disease progression, if any.
Biopsy is a procedure that extracts tissue samples from the tumour.
The trial is currently open to TNBC patients whose cancer is metastatic (when the cancer cells have spread to other parts of the body) and who wish to take part. Those interested need to fulfil certain eligibility criteria and be assessed by a doctor.
The trial is aimed at assessing the safety and efficacy of the Bexarotene-Capecitabine chemotherapy combination.
This combination was developed by clinicians and scientists from National Cancer Centre Singapore (NCCS), Singapore General Hospital and the Genome Institute of Singapore (GIS) at the Agency for Science, Technology and Research (A*Star).
Breast cancer is the most common cancer among women in Singapore, with the number of new cases increasing rapidly. From 2014 to 2018, there were 11,232 new cases of breast cancer here, exceeding the number of new cases of colon cancer in women and men in the same period.
TNBC is a more aggressive form of breast cancer compared with other subtypes and accounts for 10 per cent to 15 per cent of all breast cancers. It tests negative for the oestrogen receptor, progesterone receptor and human epithelial growth factor receptor-2, hence the "triple-negative" in its name.
Without these receptor targets, TNBC is more difficult to cure. And with limited treatment options available, chemotherapy remains the mainstay of treatment.
The risk of recurrence for TNBC is also higher compared with other breast cancer types, said Dr Elaine Lim, a senior consultant in the department of breast and gynaecology at NCCS.
"Generally speaking, there are fewer treatment options for TNBC patients whose cancer is metastatic, compared with those with breast cancer of other subtypes," she said. "Bexmet applies a novel concept of changing cell states, in order to increase the susceptibility to available chemotherapy.
"We want to see if the breast cancer cells will exhibit the same kind of molecular changes as were observed in our pre-clinical work."
The development of oncology drugs is costly, and affordability and accessibility can be a challenge. Hence, altering cancer cell states to make them more susceptible to available chemotherapy may be a cost-effective way to treat TNBC and potentially other cancers.
Currently, immunotherapy is a treatment option for TNBC patients. This involves drugs that employ the patient's own immune system to fight the cancer.
But it is expensive and a patient is likely to need multiple sessions, said Dr Lim, who is the clinical trial lead.
 A cycle of the Bexarotene-Capecitabine treatment is expected to be much more affordable, given the lower costs of Bexarotene and Capecitabine.
A cycle of the Bexarotene-Capecitabine treatment is expected to be much more affordable, given the lower costs of Bexarotene and Capecitabine.
Outside Singapore, Bexarotene is used to treat cutaneous T-cell lymphoma - a rare type of cancer that begins in white blood cells called T cells.
TNBC is generally detected either through screening or when a woman discovers a lump in her breasts - a symptom of breast cancer.
"The strongest risk factor for developing triple-negative breast cancer is genetic predisposition, such as carrying an inherited BRCA1 gene mutation," said Dr Tam Wai Leong, associate director and group leader of GIS' Laboratory of Translational Cancer Biology.
"Other contributory risk factors include family history and perhaps lifestyle-related factors, such as obesity, which are less well defined."
Bexmet is supported by the National Research Foundation and administered by the Ministry of Health's National Medical Research Council. Pre-clinical work was funded by A*Star, the council and the National Cancer Centre Research Fund.
Tags:
;
;
;
;
Internal;News Article;
;
SingHealth;SingHealth Duke-NUS Academic Medical Centre;
Article;
Tomorrow's Medicine;The Straits Times;
;
;
;
;
Tomorrow's Medicine;Research
SOURCE: THE STRAITS TIMES SINGAPORE PRESS HOLDINGS LIMITED, REPRODUCED WITH PERMISSION
