IMPROVING OUTCOMES FOR LIVER CANCER
A first-of-its-kind cohort study has been launched to diagnose hepatocellular carcinoma (HCC) more accurately at an earlier stage in high-risk patients, and to predict an individual’s likelihood of developing it.
While potentially curative treatment is possible with early diagnosis, only 20% of cases are detected at a stage where cure is possible. The ELEGANCE* study, led by the National Cancer Centre of Singapore, addresses this urgent need for high-risk individuals and aims to develop a simpler but more accurate blood test for the early diagnosis of HCC that can be done at Family Medicine clinics – to replace the current dual modalities of 6-monthly ultrasound examination and serum alpha-fetoprotein.
*The Early Detection of HCC: miRNA, Microbiome and Imaging Biomarkers in the Evolution of Chronic Liver Disease in a High-Risk Prospective Cohort
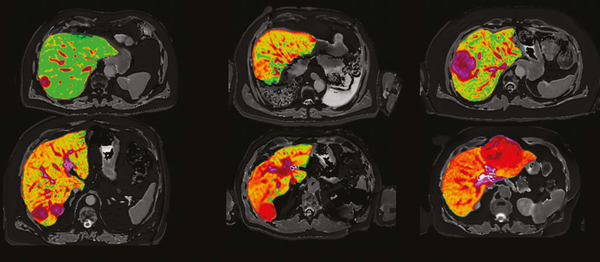
Quantitative magnetic resonance images of the liver from six patients presenting with liver cancer produced by Perspectum’s LiverMultiScan, which will be used in a new study led by National Cancer Centre Singapore
Image credit: Mole DJ et al. Plos One. 2020;15(12):e0238568
AIMS OF THE STUDY
The four-year-long study aims to enrol 2,000 participants at high risk of HCC to develop new tools for earlier diagnosis.
The study aims to improve patient outcomes by:
Developing more accurate diagnostics for early HCC
Developing an AI algorithm to predict an individual’s risk of developing HCC
Discovering novel molecular targets to prevent the development of HCC
WHAT THE STUDY ENTAILS
Enrolled patients will be monitored regularly by current standard-of-care imaging and blood tests.
The collection of additional clinical data, bio samples and regular follow-ups will continue at participating hospitals and clinics for up to three years after enrolment.
There will be no interventional treatment as this is an observational research study.
Patients diagnosed with liver cancer can continue to participate in the study and will be referred for treatment at healthcare institutions according to standard clinical practice. The cost for this treatment will not be borne by the study.
HOW AND WHO GPS CAN RECOMMEND FOR THE STUDY
The research team is actively recruiting patients with chronic liver disease as validated by blood tests and imaging.
We invite family medicine physicians to recommend eligible patients to call the study hotline at 6326 6573.
Eligibility criteria
Has chronic liver disease (e.g., liver cirrhosis, hepatitis B or C, non-alcoholic fatty liver disease [NAFLD], non-alcoholic steatohepatitis [NASH])
An ultrasound report / computed tomography scan / magnetic resonance imaging scan showing no HCC in the past 3 months
Normal alpha-fetoprotein (AFP) test results in the past 3 months
Enrolment process
Family medicine physicians who would like to find out more about the study, please contact the study’s coordinators at:
Tel: 6326 6573
Email: ahcc10@nccs.com.sg
Tags:
ABDOMEN;Liver
;
;
ABDOMEN;Liver;
;
News Article;
National Cancer Centre Singapore;
National Cancer Centre Singapore;
Article;
Defining Med;Medical News;
;
;
;
;
Defining Med;Medical News (SingHealth)
